Cathodic protection (Eng)
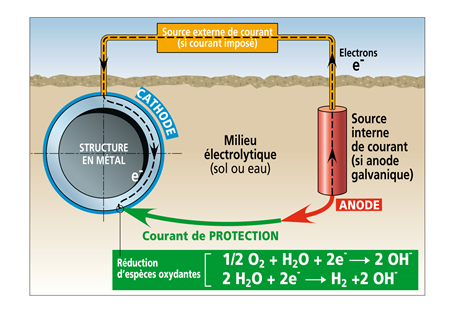
Cathodic protection is a technique enabling to reduce the corrosion rate of a metallic material, in an aqueous environment, by decreasing the corrosion potential of metal (cathodic polarization hence the term cathodic protection . The metal structure to be protected is then placed at a potential so that the corrosion rate becomes acceptable on all the metal surface in contact with the aqueous environment. For that an electrical current flows between an auxiliary anode and the material to be protected which constitutes the cathode. This current which thus flows from the environment towards metal is adjusted in order to reach a potential value for which the corrosion rate of metal becomes very low. This protection applies to any metal structure in contact with an aqueous environment in particular the carbon steel structures buried or immersed, the internal surfaces of metal capacities with an electrolyte, as well as the reinforced concrete rebars. This protection is often associated with prevention processes of corrosion such as coatings (painting, polymers...).
The cathodic protection current can be applied:
- with an external D.C generator connected between the structure to be protected (cathode) and an auxiliary anode (called current groundbed) using any conductive material (preferably rust-proof): protection by impressed current;
- by galvanic coupling between the structure to be protected and anodes made with a less noble metal or alloy than the metal we want to protect: protection by galvanic anodes.
Whatever the system, the protection efficiency depends on the density of cathodic current (and thus on the reached electrode potential) on the metal surface we want to protect. It is the obtained value of the potential which is used as a criterion to estimate the protection efficiency.


